For months, Taylor Lambert had been experiencing vague health issues: unexplained weight gain, headaches, and flu-like symptoms. Then, shortly before her 20th birthday, “I woke up one morning and I couldn’t use half of my face,” she says. Her doctors diagnosed Bell’s palsy and prescribed steroids, which helped for about a month. Then the symptoms returned.
“I went back to the doctor, and they recommended a CT scan,” says Lambert. “That’s when they found my tumor.”
Lambert was diagnosed with a golf ball-sized pilocytic astrocytoma. Though benign, this rare tumor can grow and cause serious problems by pushing into structures in the brain. Her tumor was causing pressure on her pituitary gland, which regulates hormones and reproduction, and the hippocampus, an area of the brain responsible for learning and memory.
Surgery and laser ablation removed 99 percent of the tumor. But within a few years, it had grown back. This time, her University of Utah Health doctors recommended a new treatment that would lower the chance of damage to healthy brain tissue: proton therapy.
When it opened in 2021, the Senator Orrin G. Hatch Proton Therapy Center at the U’s Huntsman Cancer Institute became one of just 37 centers in the country to offer this cutting-edge treatment to patients who could suffer negative long-term effects from traditional radiation therapy. The center is named for the former U.S. legislator in honor of his longtime support of HCI.
Prior to this, if you were to look at a map of proton centers, “There was this big void in the Mountain West,” says pediatric radiation oncologist Matthew Poppe. “We’ve been sending more and more of our patients to proton centers outside the state.”
Proton therapy is becoming the standard of care for children and young adults because it delivers less radiation to healthy tissue, Poppe says. That means a lower chance of secondary cancers or health issues later in life.
Studies undertaken prior to the wider adoption of proton therapy found that years after cancer treatment had ended, three out of five childhood cancer patients developed a health problem related to the treatment, including heart damage, infertility, thyroid problems, and cognitive issues. In addition, childhood cancer survivors have been more than twice as likely as the general population to develop a secondary cancer after age 40, according to a 2015 review of Childhood Cancer Survivor Study data.
“My doctors were worried that with [traditional] radiation blasting through both of those areas of my brain, I could have potential fertility issues as well as short-term memory issues down the road,” Lambert says. A newlywed in her early 20s, she doesn’t have kids yet. “I don’t want that option taken away from me.”
Until recently, the nearest proton therapy center was in Phoenix or San Diego. Relocating for the six-week course of treatment would have required Lambert to put her career as a muralist, painter, and special effects artist on hold. Her husband would have had to quit his job as well. But at the same time Lambert’s doctor prescribed radiation, the proton center was opening, and she was able to receive treatment in Utah. “We wouldn’t have had a source of income in another state. We wouldn’t have had a place to stay,” she says. “I’m lucky I was just in time.”
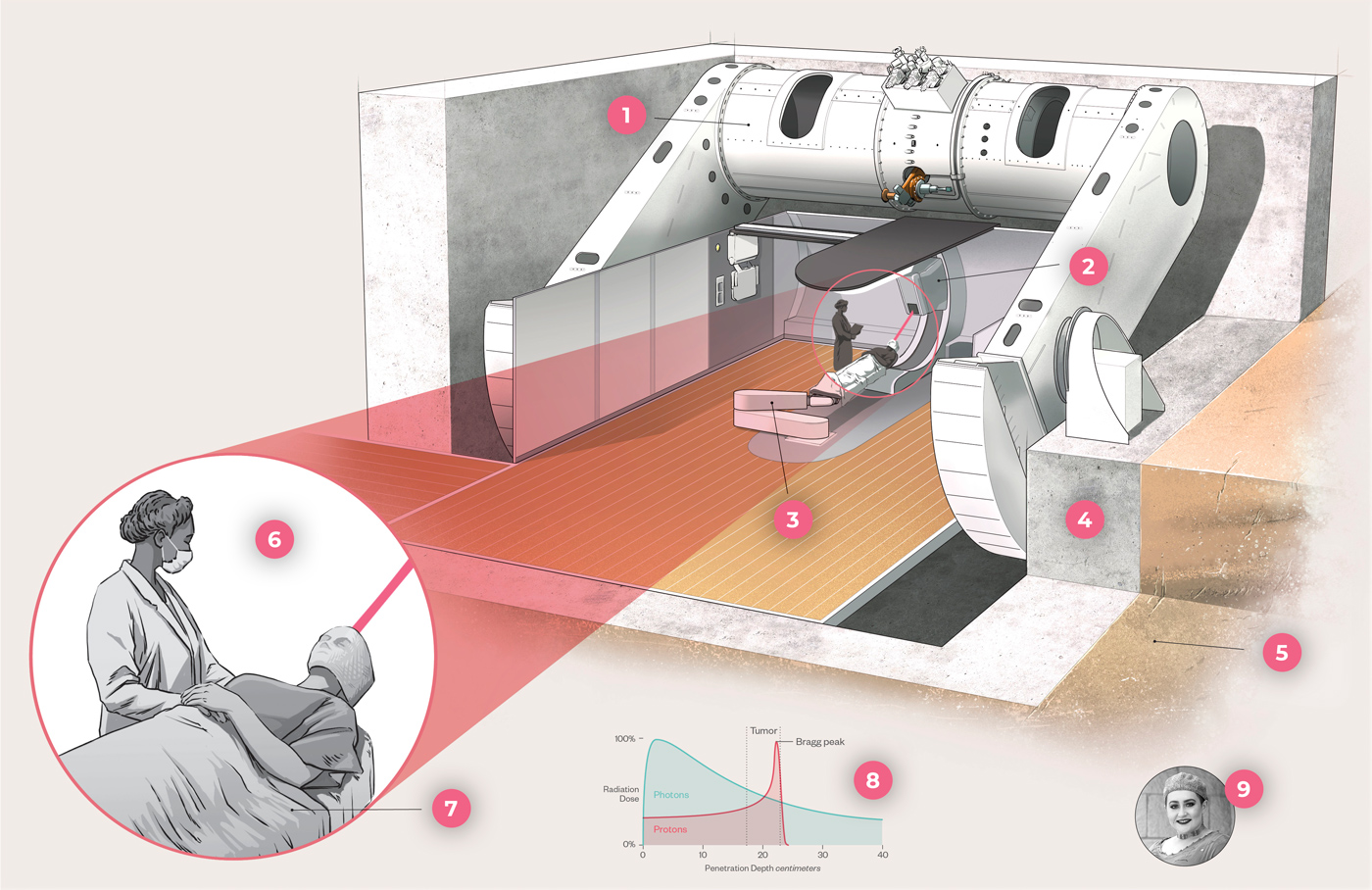
1. Proton accelerator
2. Gantry rotates around patient to direct beams.
3. A robotic table called a treatment couch moves the patient to the exact position under the gantry for proton beam delivery.
4. Six-foot-thick walls contain radiation within the vault.
5. To make room for the proton center, crews excavated several tons of rock and dirt.
6. Proton radiation beams must be delivered precisely, down to the millimeter.
7. A custom body mold and mesh face mask keep the patient still during each treatment session.
8. As photons travel, they deposit less and less energy. Proton beams deposit almost all their energy at once before stopping, a property called the Bragg peak.
9. Taylor Lambert was one of the first patients to use HCI's new proton therapy to treat a golf ball-sized brain tumor.
Eliminating the Exit Dose
Cancer treatment is a tricky balancing act: damage as much cancer as you can without making the cure worse than the disease. After X-rays were discovered in 1895, the medical world learned that radiation could shrink tumors. But physicians soon discovered it could cause the very disease it was treating when radiologists who had tested dose strength on their arms later developed leukemia.
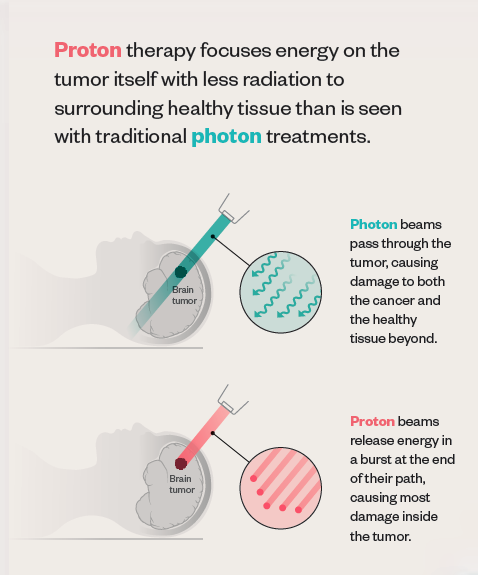 Traditional radiation uses photons, packets of energy that cause damage to all the cells they encounter along their path, with no way to control where that path ends. “After it passes through the tumor, the photon beam continues on out of the patient, depositing some ‘exit dose’ in healthy tissue,” explains Bill Salter, professor and chief of the Division of Medical Physics in the U’s School of Medicine and senior director of radiation oncology at HCI.
Traditional radiation uses photons, packets of energy that cause damage to all the cells they encounter along their path, with no way to control where that path ends. “After it passes through the tumor, the photon beam continues on out of the patient, depositing some ‘exit dose’ in healthy tissue,” explains Bill Salter, professor and chief of the Division of Medical Physics in the U’s School of Medicine and senior director of radiation oncology at HCI.
Since the turn of the 20th century, scientists have known that protons—particles inside atoms—travel a finite depth and deposit most of their energy at the end of their path, a property known as the Bragg peak. In 1946, physicist Robert Wilson published a paper proposing the use of protons in medical treatments. Because of the Bragg peak, Wilson pointed out, proton beams can travel to a tumor, deliver the dose of radiation, and then stop. In other words, no exit dose.
Proton therapy isn’t necessarily more effective at killing cancer cells. The true benefit comes from that lack of exit dose. “We use protons because that does a better job of decreasing and preventing some of the long-term toxicities of radiation,” says Poppe.
Still, most HCI patients who need radiation will continue to receive traditional photon therapy, “not because we don’t have capacity to treat them with protons, but because there’s not a significant benefit,” he notes. “Each case needs to be looked at on an individual basis.”
Life expectancy is one factor physicians consider, and young adults and children are good candidates since late effects or secondary cancers may occur several years down the road. Tumor location also factors in. Proton therapy may be recommended if the cancer is near an area of the body especially sensitive to radiation.
Proton therapy sessions are similar to traditional radiation. A typical course may involve daily 30-minute treatments for a period of several weeks. During treatment, a machine called a cyclotron accelerates protons to two-thirds the speed of light. A large structure called the gantry rotates the radiation beams around the patient, who typically lies in a custom-made body mold to help maintain accurate positioning as the beams enter the body at different angles.
Whether proton or photon, radiation beams don’t hurt when they enter the body. And short-term side effects—most commonly, nausea, vomiting, diarrhea, fatigue, and skin changes—are similar for both types of radiation therapy.
HCI now houses the only proton therapy center in the region.
Moving Mountains
Though Wilson’s research was published in 1946, it wasn’t until 1990 that the first hospital-based proton therapy treatment center opened. Costly equipment and limited technology kept proton therapy on the fringes.
As technology improved and the price of equipment decreased over the decades, administering proton therapy became more feasible. But adding a proton facility is still a major undertaking. Most centers are located in big cities and are accordingly built for a large population. They typically contain four or five gantry systems, taking up the space of a football field and costing up to $250 million.
“That would not be sustainable here and would be more than we need,” says Poppe. “We were waiting for the right technology for our patient population.”
In 2012, the FDA approved the use of a compact proton therapy machine that contains a smaller, built-in cyclotron. HCI opted to install one unit, bringing the total cost of the facility to $31 million. The site contains enough room for a second machine if needed down the road. But for now, U director of radiation oncology Salter says, “We’re trying to take a reasonable bite.”
Despite having very little room to expand—HCI sits so close to the foothills that rattlesnakes and tarantulas sometimes grace the premises—leaders were committed to building the proton therapy center onsite.
“We didn’t want to split our team of experts up,” says Salter, who oversaw the building process. “We wanted those experts to be available to care for the patient, day in and day out.”
All they needed was to find an unused corner of the property to squeeze in the 7,450- square-foot building. That space was a small section between the cancer hospital and research building.
“It’s a 25-degree slope on the side of a hill, which is why it was unused,” Salter laughs. “But if we were to move a piece of that mountain, we would have a perfect spot to put this proton unit.”

In order for construction equipment to even get to the space, crews had to build a temporary road behind the HCI research building, putting in switchbacks due to the steep grade of the hill. Then came the process of digging up and moving hundreds of truckloads of mountain—not just dirt, but also rock. Blasting was not an ideal option. “We’re right here next to a research facility, which is very sensitive to vibrations,” notes Salter. The team had to come up with alternatives—a giant mechanical jackhammer to break up the large boulders or an expanding chemical material that breaks rock.
“In the end, with much persistence, the giant jackhammer did the trick,” says Salter.
While crews worked through construction hurdles, the radiation oncology team faced its own obstacle of undertaking a two-year training process. Instead of recruiting from other proton centers, leaders wanted HCI’s existing medical physicists, physicians, radiation therapy technicians, and other members of its already talented team to learn the necessary skills.
Then came COVID-19. “We had to change the original plan radically,” says Salter. “We were forced to cancel visits to outside centers and switched almost exclusively to virtual interactions.”
Through phone calls, virtual trainings from the equipment vendor, and video presentations from colleagues at other proton centers, HCI’s radiation oncology team learned the nuances of using proton therapy.
“We managed to get our entire team trained up in that environment,” Salter says. “We must be the only proton center in the country to go live during a pandemic.”
The Cancer Center of the Mountain West
An estimated 100 to 200 patients per year will receive treatment at HCI’s proton therapy center. But the impact of the facility extends beyond those patient numbers, Salter and Poppe say.
“I think our prominence in the field and in pediatric cancer care will only continue to grow over the next five to 10 years, in part because of this addition,” says Poppe. “HCI and Primary Children’s Hospital together are becoming one of the premier pediatric cancer centers in the Mountain West.” While children receive most of their cancer care at Primary Children’s, their radiation therapy is provided at HCI.
With patients coming from Idaho, Montana, Wyoming, and Nevada, as well as Utah—a geographic region that spans 17 percent of the continental U.S.—HCI serves as a cancer treatment mecca for a largely rural area.
“We are certainly not the biggest cancer center in the world, but the addition of protons to our arsenal means we are as well-equipped as any cancer center anywhere,” says Salter. “When a patient comes here, that patient can know they’re getting access to the best tool for their particular tumor.”
Lambert says she feels fortunate to have this treatment available in her own backyard. “It’s so convenient to drive just 45 minutes, get treated, then get back to doing a mural,” she notes. “It allows me to focus on what I do, rather than taking weeks and months out of my life to travel somewhere.”
Knowing she has a lower chance of tumor growth and other problems down the road brings Lambert peace of mind. And recently, she and her husband began talking about having a family. “With proton therapy, the chance of fertility problems was significantly reduced. It could mean the difference between having children and not,” she says. “That gives me hope for the future.”
About the author: Lisa Anderson is a writer and editor for Huntsman Cancer Institute.



Comments
Comments are moderated, so there may be a slight delay. Those that are off-topic or deemed inappropriate may not be posted. Your email address will not be published. Required fields are marked with an asterisk (*).